While Lithium-oxygen is the ultimate prize for high energy density-seeking battery companies, it has severe issues with power capability -- especially since you need to force the fleeting gas (oxygen from air) to react fast enough to keep up with state-of-the-art solid state batteries. This issue made everybody look at sulfur, which does not have the concentration problem, being a solid and otherwise similar to oxygen in reactivity. Sulfur enables slightly less energy density because it is a less aggressive non metal (so voltage would be 2.4V instead of 3V), and it is lightly heavier because of its position on the third row of the periodic table. However, a Li / sulfur battery theoretically offers 2550 Wh/kg, which is more than five times higher than the best available Li-ion battery. This is a prize worth pursuing. And for the past 30 years many companies have attempted to create batteries with high densities. But like with all promising technologies, there are lots of hurdles – if there was none, it would have been done years ago. The trick is to attempt to commercialize a new chemistry at the right time – when material science has offered just the right new tools to solve the old problems, and at the same time the demand is so high that it can justify higher price for higher performance.
Is now such time for Li / sulfur? It remains to be seen, but let's look at the actual problems and new solutions. And maybe we can make an educated guess on chances of overcoming them.
Low conductivity. To provide electron flow active material needs to be electrically conductive. Sulfur is an insulator, so it need to be mixed with some conductive additive to bring electron flow very close to its surface where reaction will take place. The more additive the better the conductivity, and also the higher the contact area with sulfur, which speeds up charge transfer reaction so power capability increases. Unfortunately this conductive matrix occupies some volume and has some weight, which cuts into the energy density. In fact, the best commercial Li/S batteries (Sion Power) that have decent power capability are at 350Wh/kg, which is more than seven times less the theoretical level and does not even beat traditional LiCoO2 based cells. New tools to solve the problem? Nanotechnology. By making the matrix out of material specially shaped on nano-scale, they can retain high conductivity and high surface area needed for wide contact with sulfur while having itself low profile and weight. The kind of nano-structures considered vary widely. Carbon nano-wires are popular because they have great conductivity along their length, while keeping weight to the minimum. Carbon foams and graphene “petals” that resemble flowers are popular to increase surface area. Sulfur itself is being prepared as nano-sized particles or films deposited on carbon structures.
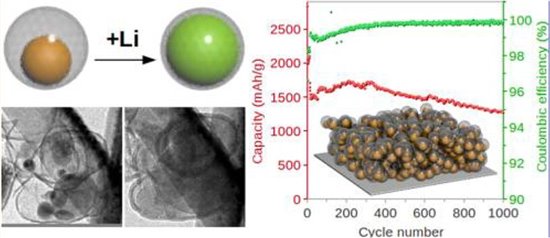
Sulfur solubility in the solvent. In a battery it is desirable that cathode material would stay where it is -- attached to positive current collector. If it can freely wander through the cell, it can contact the anode material directly and wastes the energy by reacting with it instead of passing the electrons over the external circuit. Unfortunately that is exactly what happens with sulfur cathode because sulfur atoms tend to make chains, circles, etc. with each other, which causes it to react with dissolved reduced product Li2S by forming polysulfide with general formula L2(Sn) where n can be very large number. This way sulfur is carried to various places in the cell and can be deposited far away from the carefully designed carbon structures that are supposed to contact it. This way sulfur is lost, and energy can not longer be extracted from the cell. For this reason even the best Li/S batteries typically show only 300 cycles vs 500-1000 cycles of rational Li-ion. To add insult to injury, sulfur can even be deposited very close to Li-anode reacting with it directly and creating potentially unsafe mix. What can be done with it? Again nano-structures to the rescue. A popular solution proposed by Prof. Yi Cui group is to put active material, such as sulfur or silicon as a “yolk” in the middle of oversized “eggshell” of conductive protective layer. When “yolk” is charged it is growing just like the chick embrion in an egg, but still has enough space without cracking the shell. The shell prevents polysulfides from leaking into the solution while also providing close electric contact. A similar solution is to pack sulfur inside carbon nano-tubs so that when it expands in stays inside like meat inside a sausage.
Fig.1 A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes, Nian Liu, Hui Wu,⊥ Matthew T. McDowell, Yan Yao, Chongmin Wang, and Yi Cui
Lithium safety. With everybody concerned about the safety of present day Li-ion cells, we are forgetting that Li-intercalation graphite anode itself was invented as a safe replacement for highly unstable Li-metal anode. After Moly Energy Li-metal battery factory fire in the nineties, no serious mass manufacturer was considering making a Li-metal rechargeable battery. Li-dendrites growth during charge is too unpredictable and can short the electrodes with catastrophic results. Luckily, polysulfides have an unexpected side effect of shaving off the growing Li-dendrites by reacting with them, and passivating the Li surface with solid electron-insulating Li2S layer that is mechanically strong and even stopping short-circuits under mechanical cutting or piercing the battery. Unfortunately the same layer makes their internal resistance quite high, cutting down battery power capability. Better solution would be to replace Li-anode completely with something more safe yet higher energy than tradition graphite, like Si-anode. But in this case we would need to have some source of lithium in the system. One way is to assemble cathode in discharged state as Li2S but it is difficult to deposit it in nano-shape since it is highly unstable in presence of water. Another approach is to still use much cheaper elemental sulfur, but add lithium in form of passivated lithium powder (such as provided by FMC corporation) to either anode or cathode and let it react on the spot once electrolyte is filled. This is an innovative approach that might help with many new interesting materials that could provide large energy but don’t come as convenient Li-containing compound.
Solvent flammability. Like all other high-energy batteries, Li/sulfur would have to use organic solvent, since water is violently reacting with lithium. Unfortunately all organic solvents are flammable, which is already a concern with present day Li-ion batteries but will be even more a concern when Li-metal is used. Interesting approach is to get rid of solvent completely and just use a very thin layer of solid electrolyte between anode and cathode. Solid electrolyte is a ceramic and cannot burn at all! More and more solid state materials that can conduct Li is being discovered, and this is an exciting area of research for any kind of Li-ion battery but particularly for Li-sulfur since products of its burning are SO2 that is much more irritating than for traditional Li-ion batteries case.
Based on all above advances, when will we see Li-sulfur batteries on store shelves?
Several companies in particular Sion and OXIS are offering prototypes for testing already. First applications are likely in next few years outside of consumer space, such as grid storage, where high energy density and low cost are key, while concerns of burning sulfur in the middle of the Arizona desert are reduced. As safety is proven and device makers familiarity with this chemistry increases, I expect to eventually see them in portable devices, since five times higher energy density is just irresistible to modern ultra-compact energy hungry gadgets.
Additional Resources:
- A look at future battery chemistries blog post by Yevgen
- Check out TI's battery management chargers and capacity fuel gauges