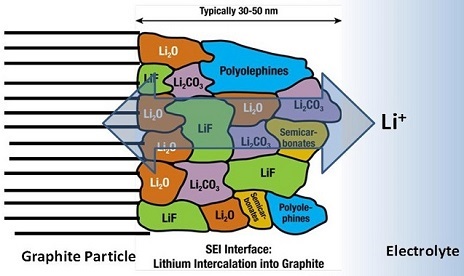
Figure 1: Solid Electrolyte Interphase (Source)
The term “solid electrolyte interphase” (SEI) often comes up in lithium-ion (Li-ion) battery literature. It is important to understand what SEI is at a high level, as this component is one of the main contributing factors to Li-ion battery aging and resistance.
Three major components of a Li-ion battery are the anode, cathode and electrolyte. During the charging process, the positive Li-ions are transferred from the cathode to the anode through the electrolyte. However, the electrolyte is electrochemically and thermodynamically unstable under the open charging voltages of Li-ion batteries. Upon contact with the Li-ions and anode electrode, the reactive elements in the electrolyte undergo reductive reactions (by accepting electrons from the anode electrode). The decomposition of electrolytes leads to the formation of SEI at the surface of the anode, accompanied by an irreversible loss of Li-ions.
A good SEI passivation layer is permeable to Li-ions and impermeable to electrolytes and electrons. This can allow reversible diffusion of Li-ions without any consumption, and effectively prevent the electrolyte from further reduction. Although SEI formation at the anode takes place mainly in the first few charge/discharge cycles, SEI continues to grow and change composition throughout the battery’s entire life.
Since SEI is made up of electrolyte decomposition products (Figure 1), the composition and properties of the electrolyte determine the formation of the SEI layer. Commonly used commercial electrolytes are carbonate-based, including ethylene carbonate (EC), ethymethene carbonate (EMC) and dimenthle carbonate (DMC). Stable SEI formation requires beneficial electrolyte properties, such as retention of the SEI interface during volume expansion and contraction of the anode and high conductivity of the Li-ions.
An effective and stable SEI is crucial to a battery’s long-term cycling performance. Degradation of SEI negatively impacts battery life. Components in SEI, such as lithium alkyl carbonates and lithium alkoxide salts, are thermally unstable and sensitive to moisture. Decomposition can cause SEI to dissolve/peel off/evolve during the cycling process, leading to further corrosion of anode material. Solvent co-intercalation, gas evolution and volume changes also accelerate the degradation process of the anode, with further growth of the SEI layer and a thicker diffusion barrier for the Li-ions. In summary, SEI formation and growth directly contribute to an increase in battery impedance, capacity fade and power fade.
Additional Resources:
- Learn more about TI’s battery management products at www.ti.com/battery
- Learn more about how TI’s gas gauges track impedance at SLUA450 – Impedance Track Algorithm Basics
- Learn more about gas gauging for lithium-ion batteries