Hello TI Technical Forum community,
I hope you are all doing well. I would like to seek your expert advice and insights regarding the use of the DRV8701 motor driver IC from Texas Instruments for a medical-related development project. However, I recently encountered a note in Altium stating that the DRV8701 is not recommended for new designs. This has raised some concerns, and I would greatly appreciate your guidance in evaluating the suitability of this IC for my application.
Given the critical nature of medical projects, it is essential to ensure the reliability, performance, and longevity of the components we choose. Therefore, I have a few questions and concerns that I would like to address:
-
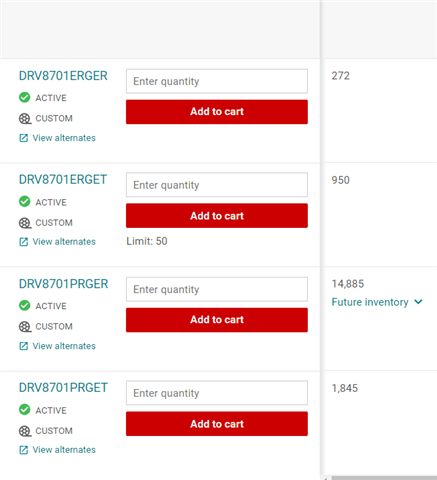
Altium's recommendation: Could someone shed light on the reasons behind Altium's recommendation of not using the DRV8701 for new designs? Are there any specific limitations, known issues, or potential risks associated with this IC that I should be aware of?
-
Medical application suitability: Considering the medical application, I am seeking insights from the community on whether the DRV8701 is a wise choice for this project. Are there any documented instances or experiences where the DRV8701 has been successfully used in similar medical applications?
-
Alternatives or recommendations: If the DRV8701 is not advisable, I would appreciate any suggestions for alternative motor driver ICs that are specifically recommended for medical use. Are there any other options available that offer better performance, reliability, or specific features relevant to medical applications?
I understand that Texas Instruments' official documentation and technical support would be the most reliable sources for comprehensive information. However, I believe that the collective knowledge and experiences of the TI Technical Forum community would be invaluable in providing practical insights and recommendations.