Other Parts Discussed in Thread: OPA2197, OPA828, LMP7721
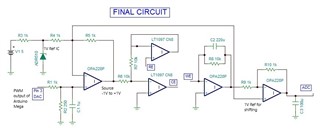
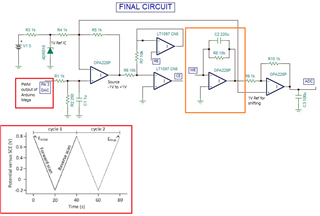
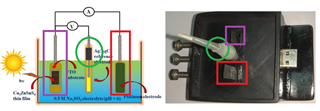
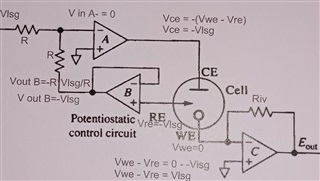
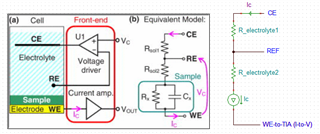
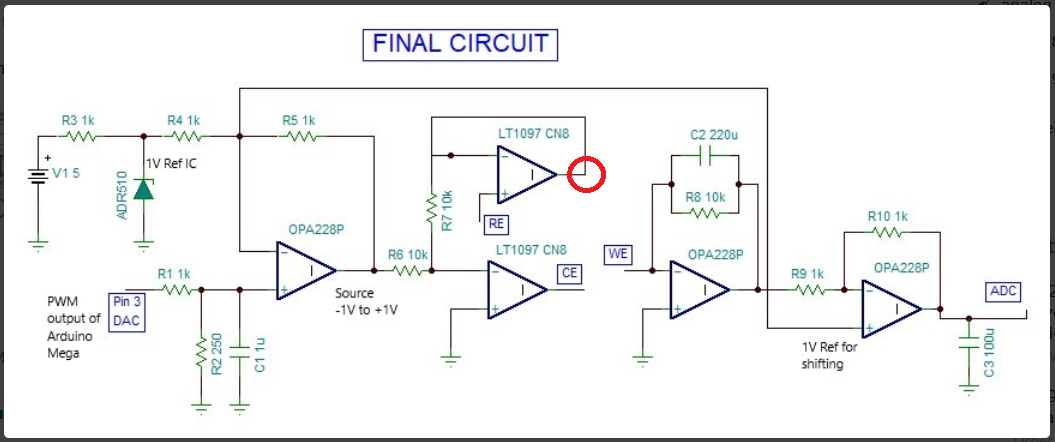
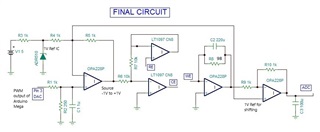
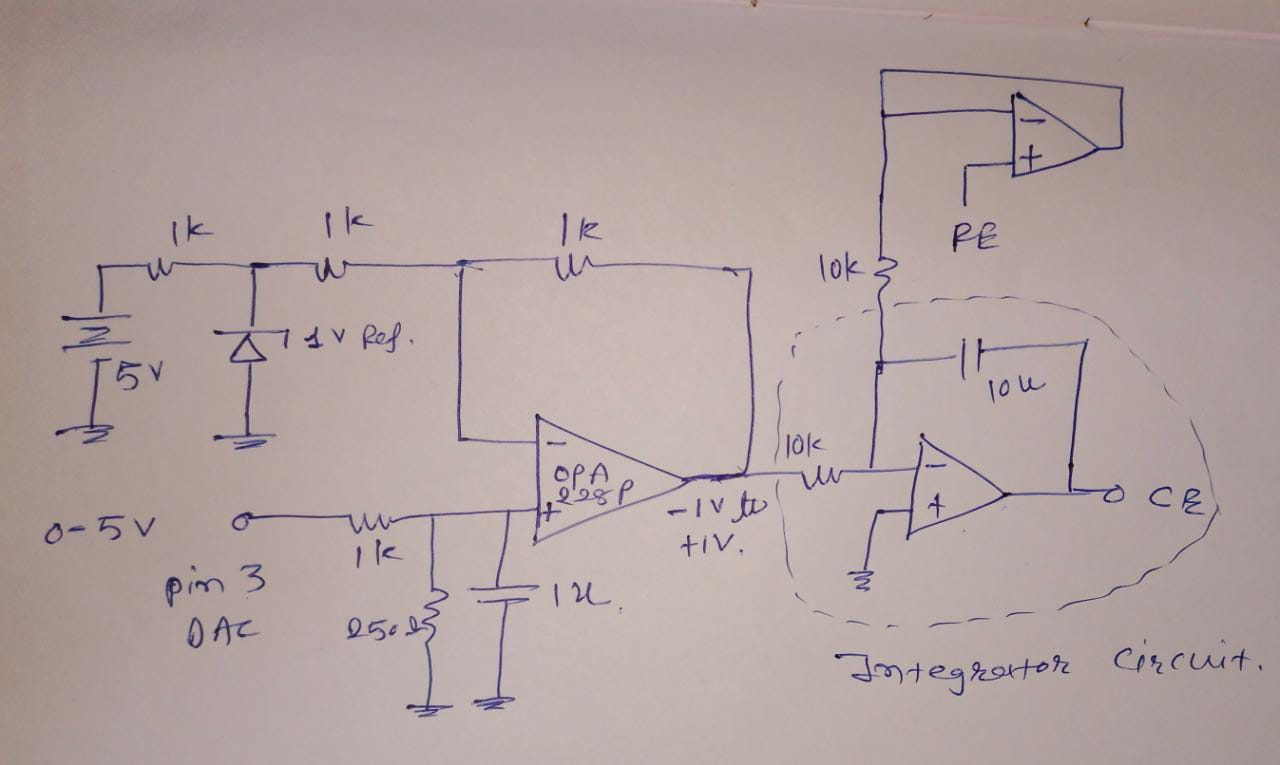
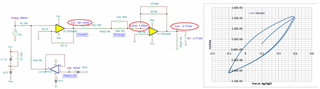
This is a circuit of cyclic voltammetry in which all three electrode i.e. WE (Working Electrode), CE(Counter Electrode) & RE (Reference Electrode) are dipped in electrolyte solution.
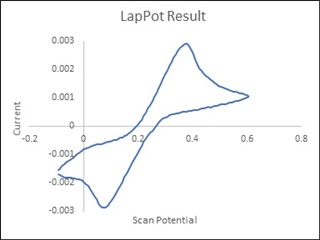
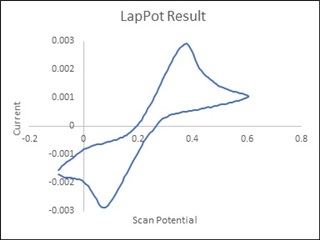
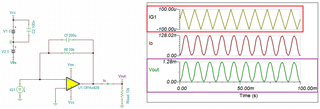
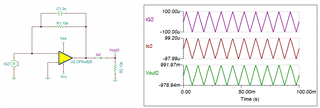
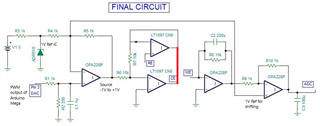
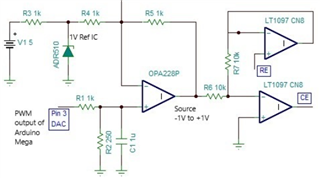
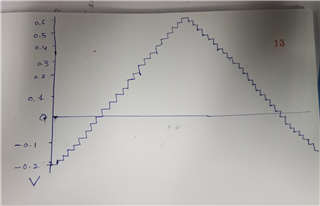
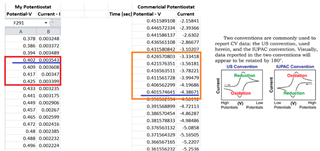
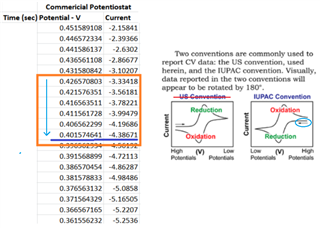
The source voltage is -1V to +1V (mentioned in circuit) but generally we are using -0.2V to +0.6V as scanning voltage. During scanning (-0.2V to +0.6V), I have measured the voltage between working electrode (WE) and reference electrode (RE) which is -0.4 to +0.8V (approx.) which should be -0.2V to +0.6V and this is happened due to electrolyte (solution resistance).
The circuit may need to improve by taking feedback from WE & RE to make it exact -0.2V to +0.6V.
I want few suggestion for circuit improvement.