Other Parts Discussed in Thread: LMP7721, LMP91000
Hello,
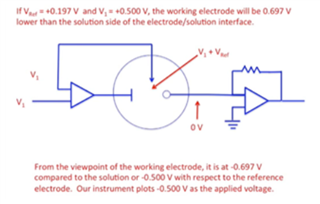
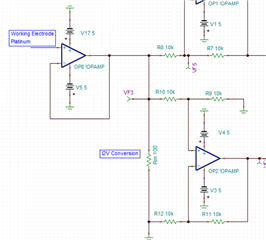
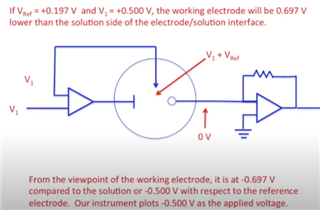
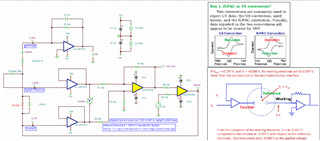
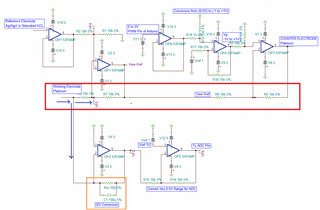
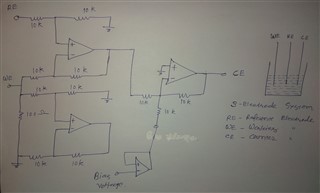
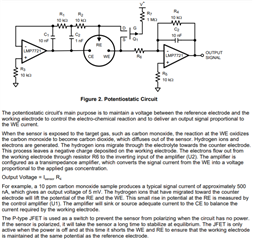
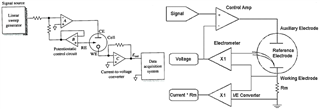
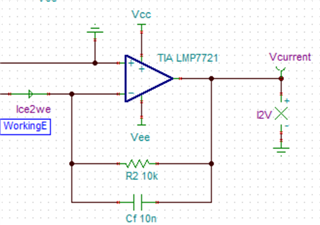
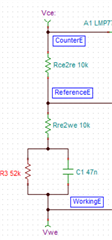
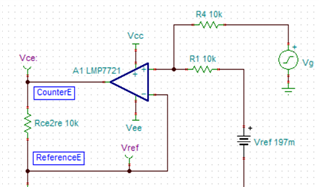
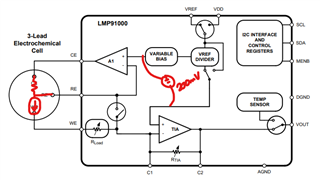
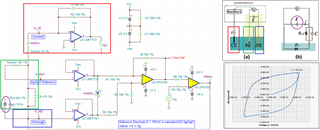
I have developed op-amp circuit for three electrode system as shown in attached image.
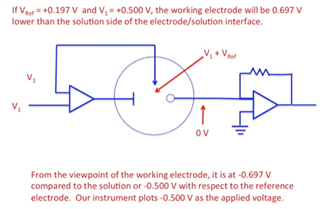
In this, I want to apply bias voltage between WE (Working electrode) and RE(Reference Electrode). All three electrodes are kept together in electrochemical cell.
In testing, I have applied 1.8V as bias voltage but no any current flow found through CE(Counter Electrode).
Actually, current should be flow through CE. Please suggest.

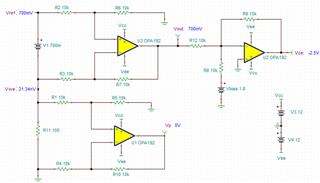

 .
. 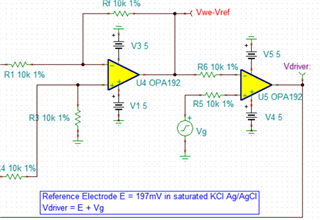 and
and